First order reaction is independent of the concentration we all know that Therefore you have to first find the rate constant by the equation T1/2= 0693/k and then put the value of rate constant (k) in integrated rate law equation ie, Ln(a°)/(a)= k*T1/2 here a° = Since the reaction order is second, the formula for t1/2 = k1A o1 This means that the half life of the reaction is seconds This means that the half life of the reaction is seconds In the reaction A 2B 2C D If the concentration of A is increased four times and B is decreased to half of its initial concentration then the rate becomes 480 ques 422 sum plz Rate of formation of so3 according to reaction 2so2=2so3 is
Solved Derive The Integrated Rate Law And The Half Life Equations For The Following Course Hero
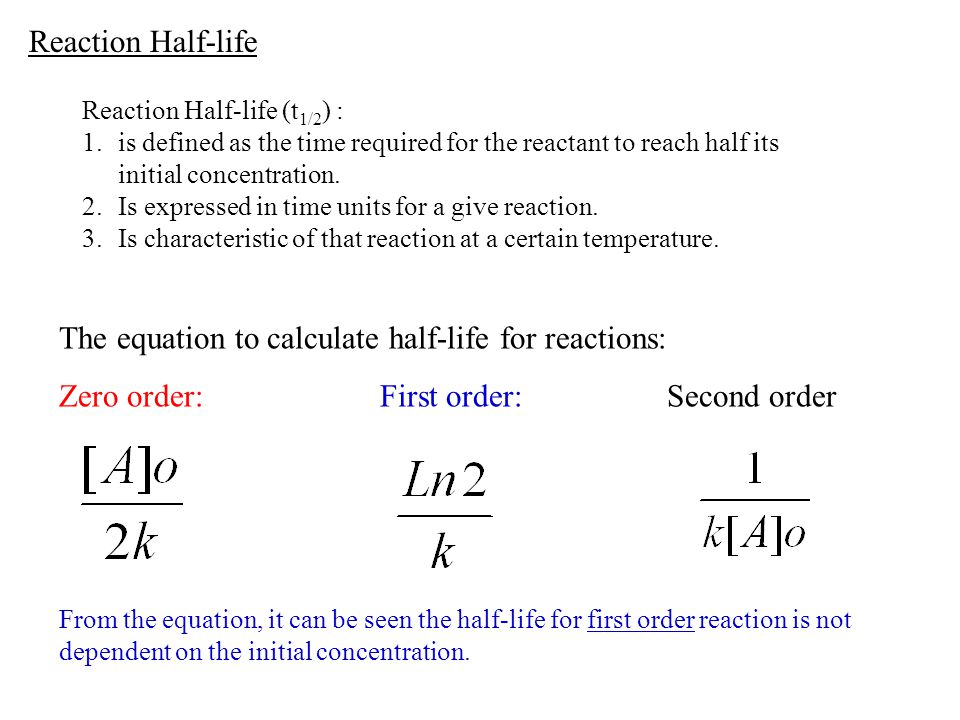
T1/2 for zero order reaction
T1/2 for zero order reaction-Are solved by group of students and teacher of NEET, which is also the largest student community of NEET A chemical reaction that is firstorder in X is observed to have a rate constant Chemistry For the reaction 2 NO (g) Cl2 (g) → 2 NOCl (g) If the concentration of NO is tripled, the rate of the reaction increases by a factor of nine If the concentration of Cl2 is cut in half, the rate of the reaction is decreased to




Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy
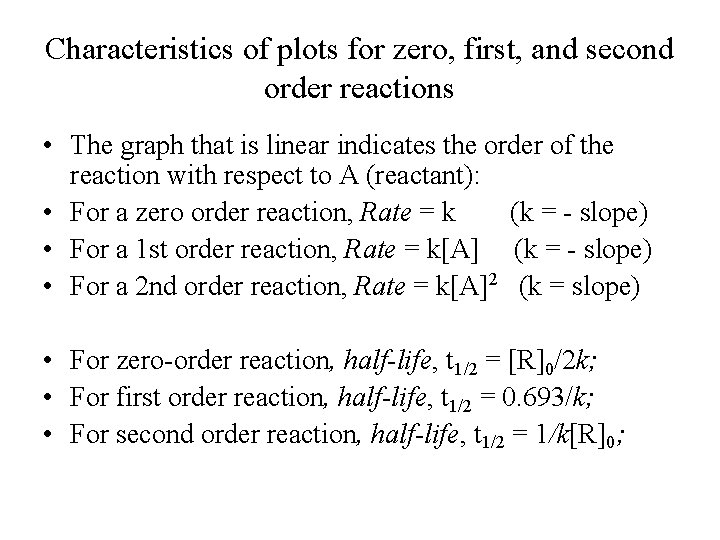
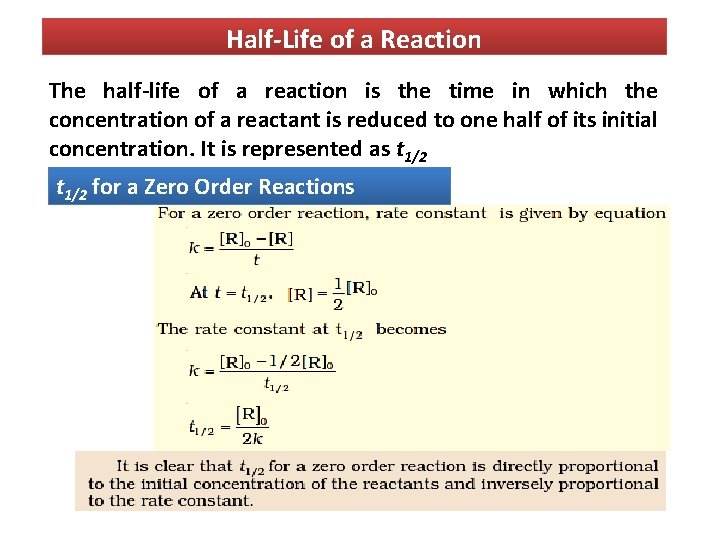
A t1/2 = A0 /2k b t1/2 = 0693/2k c t1/2 = A0 /2 d t1/2 = 2k/ A0 Answer a 4 The unit of k for zero order reaction is a moles/litre/second b moles c moles/second d moles/litre Answer a 5 Which of the following is the half life of first order reaction?T 1 / 2 = t 5 0 % ⇒ R = 2 R 0 t 1 / 2 = k R 0 − 2 R 0 = 2 k R 0 Therefore, the formula of t 1 / 2 for a zero order reaction is 2 k R 0The halflife of a reaction, t1/2 , is the time it takes for the reactant concentration A to decrease by half For example, after one halflife the concentration falls from the initial concentration A to A/2, after a second halflife to A/4, after a third halflife to A/8, and so on onFor a firstorder reaction, the halflife is constant
As the reaction progresses for zero order reactions the half life does of the reagent does indeed become shorter and shorter (ie if the concentration starts at 100moldm^3 and reaches 50moldm^3 in 10 seconds, then the concentration will half again to 25moldm^3 in just 5 more seconds and so onThe rate constant of the reaction is KEAM 15 2 t 1 / 2 for a first order reaction is 1426 m i n Calculate the time when 5 % of the reactant is left UP CPMT 15 3 The inversion of cane sugar is first order in sugar and proceeds with halflife of 600 minutes at pH =Manipal 09 t1/2 for a first order reaction is 10 min Starting with 10 M, the rate after min is (A) text M text min1 (B) × 5 Tardigrade Pricing
In case of zero order reactions a) t1/2 = 2t1/4 b) t3/4 = 3t1/2 c) t(infinity)= 2t1/2 D) All Answer is a and c are correctt t1/2 = R °/2K And t 1/4 = R °/2k HIs done on EduRev Study Group by NEET Students The Questions and Answers of The relation btw t7/8 and t1/2 for zero order reaction is explain? This discussion on The relation btw t7/8 and t1/2 for zero order reaction is explain?




1 Chapter 12 Chemical Kinetics 1 Second Order Rate Law 2 Zero Order Rate Law 3 Reaction Mechanism 4 Model For Chemical Kinetics 5 Collision 6 Catalysis Ppt Download



Chapter 16 Rate Of Reactions Kinetics Rates And Mechanisms Ppt Video Online Download
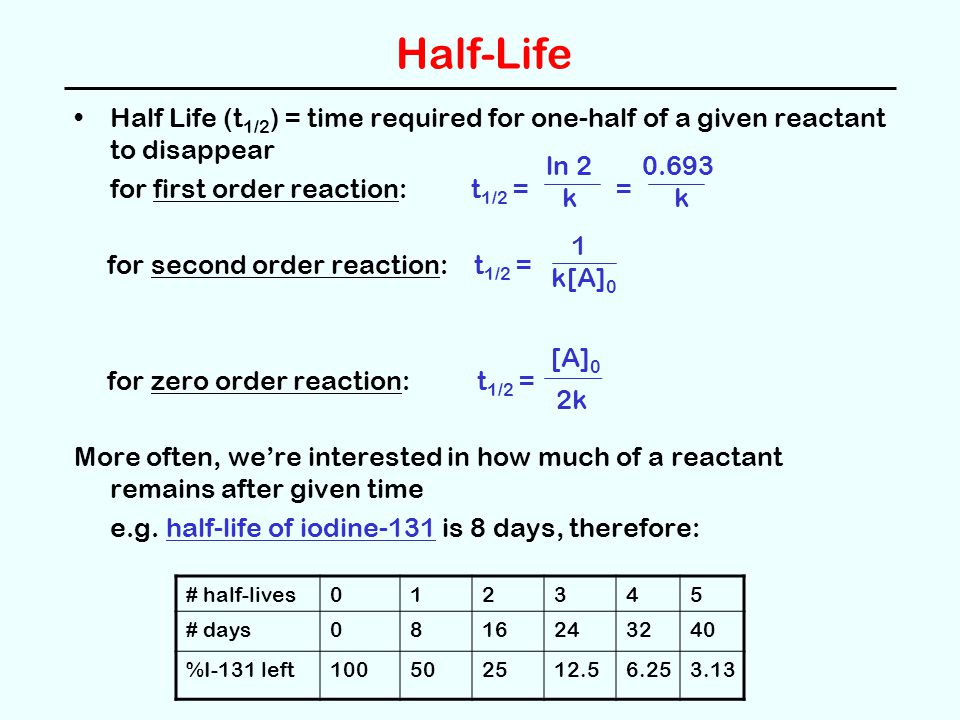
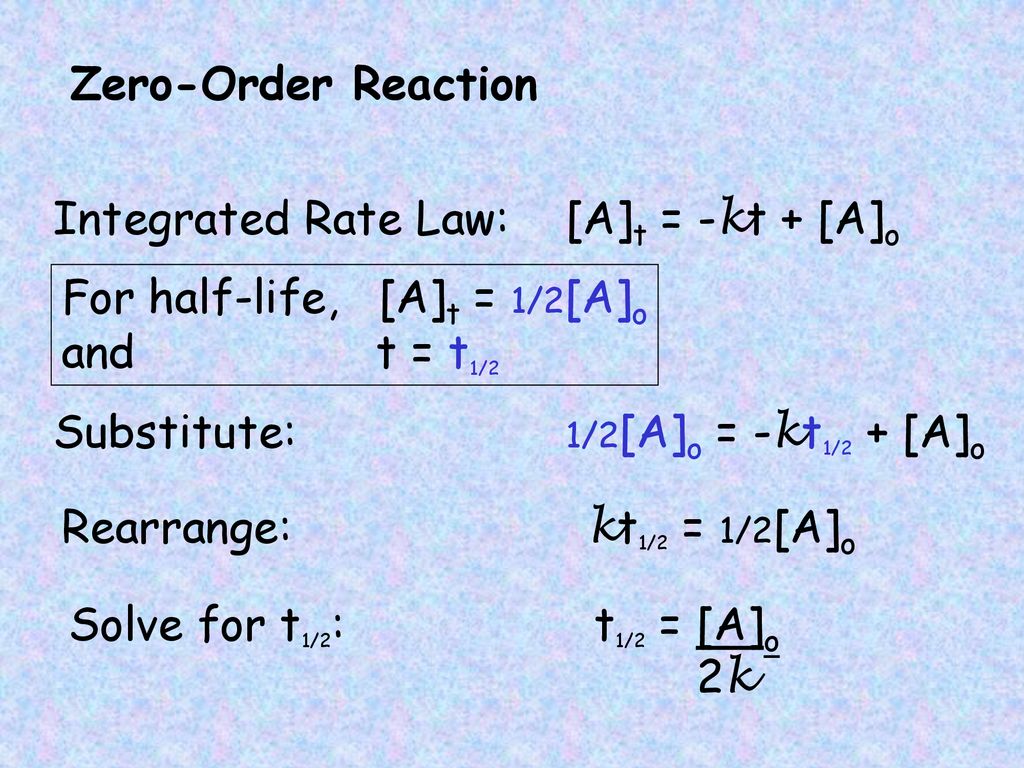
If you know the zero order kinetics then, use the formula and put the numerator inside log as I (initial concentration) and the denominator inside the log term as I/4 (initial/4) This will give you an equation for T3/4 Similarly put the denominator as I/2 for the T1/2 equationFor a zero order reaction A products , rate = k t ½ = A o / 2k For a first order reaction A products , rate = kA t ½ = 0693 / k For a second order reaction 2A products or A B products (when A = B), rate = kA 2 t ½ = 1 / k A o Top Determining a Half LifeTherefore, t 1/2 can be written as kt1/2 = 1 2A0 k t 1 / 2 = 1 2 A 0 And, t1/2 = 1 2kA0 t 1 / 2 = 1 2 k A 0 It can be noted from the equation given above that the halflife is dependent on the rate constant as well as the reactant's initial concentration




Solved What Is The Formula To Find The Value Of T1 2 For A Zero Order Reaction




The Concept Of T1 2 Is Useful For The Reactions Of
A t1/2 = A0 / 2k b t1/2 = 0693 / 2k c tl/2 = 2kClick here👆to get an answer to your question ️ What is the formula to find the value of t1/2 for a zero order reaction? 3 Which of the following is the half life of zero order reaction?



What Is The Relation Between T1 2 And T3 4 For Zero Order Reaction Quora




Examine The Half Life Equations Below To Answer The Chegg Com
Contributors and Attributions The halflife of a reaction ( t1 / 2 ), is the amount of time needed for a reactant concentration to decrease by half compared to its initial concentration Its application is used in chemistry and medicine to predict the concentration of"t1/2 will be higher" higher than what exactly!




Using The First Order Integrated Rate Law And Half Life Equations Worked Example Video Khan Academy



1




T1 2 Is Inversely Proportional To Initial Concentration In Zero Order Reaction True False Brainly In




Pubdoc 11 348




For A Zero Order Reaction A Rarr B T 1 2 Is K Is Rate Constant



Solved Derive The Integrated Rate Law And The Half Life Equations For The Following Course Hero




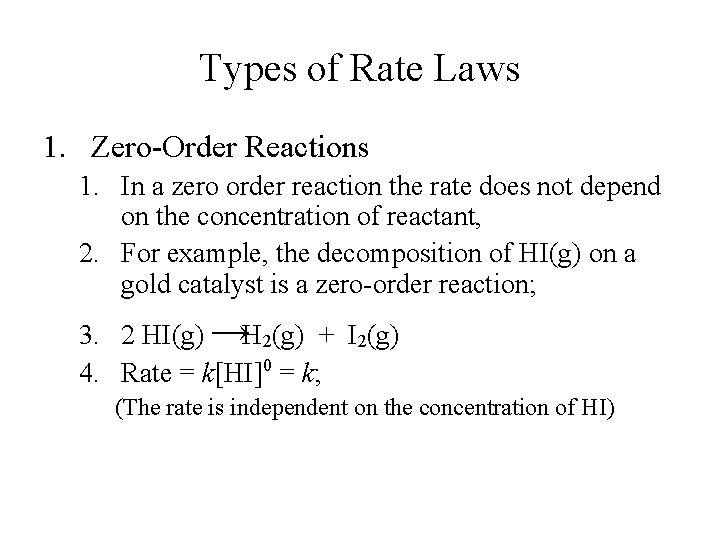
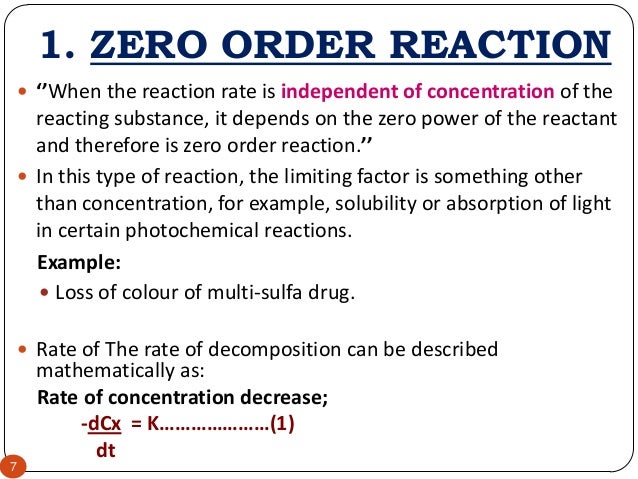
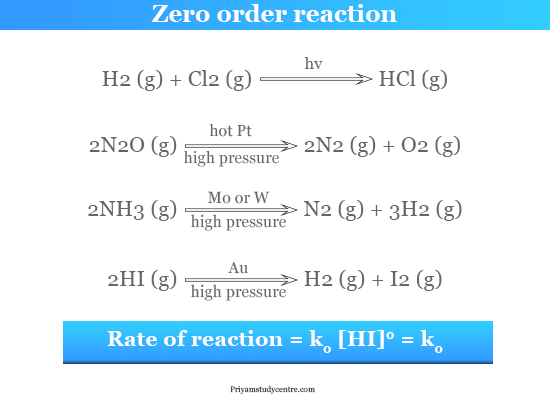
Zero Order Reaction Definition Examples Formula



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of




Rate Equation Wikipedia




51 Which Of The Following Represents The Equation For A First Order Half Life A 1 2 Homeworklib



Half Lives



14 4 The Change Of Concentration With Time Integrated Rate Laws Chemistry Libretexts




What Is The Ratio Of T3 4 T1 2 For A First Order Reaction Chemistry Topperlearning Com Qfujetmm




Which Of The Following Graphs Formed Plotted Between T 1 2




Column I Column Ii P Zero Order Reaction 1 T
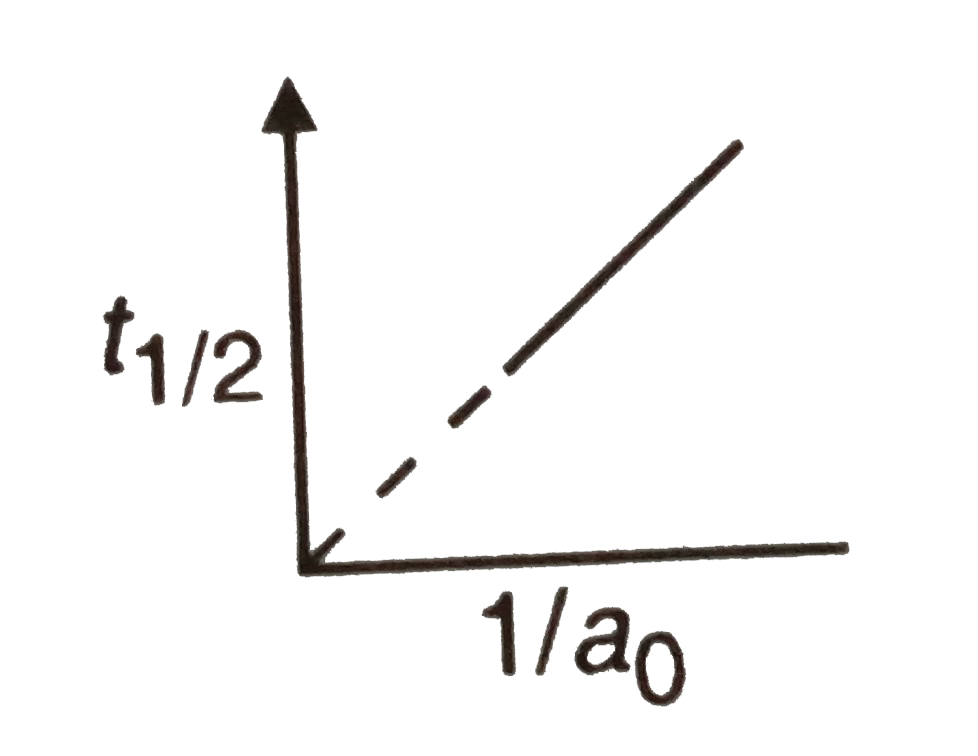



The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png



For A Certain First Order Reaction T1 2 100 Sec How Long Will It Take For The Reaction To Complete 75 Quora




Chemical Kinetics And Stability The Purpose Of Stability




Quantitative Pk I Ii Flashcards Quizlet
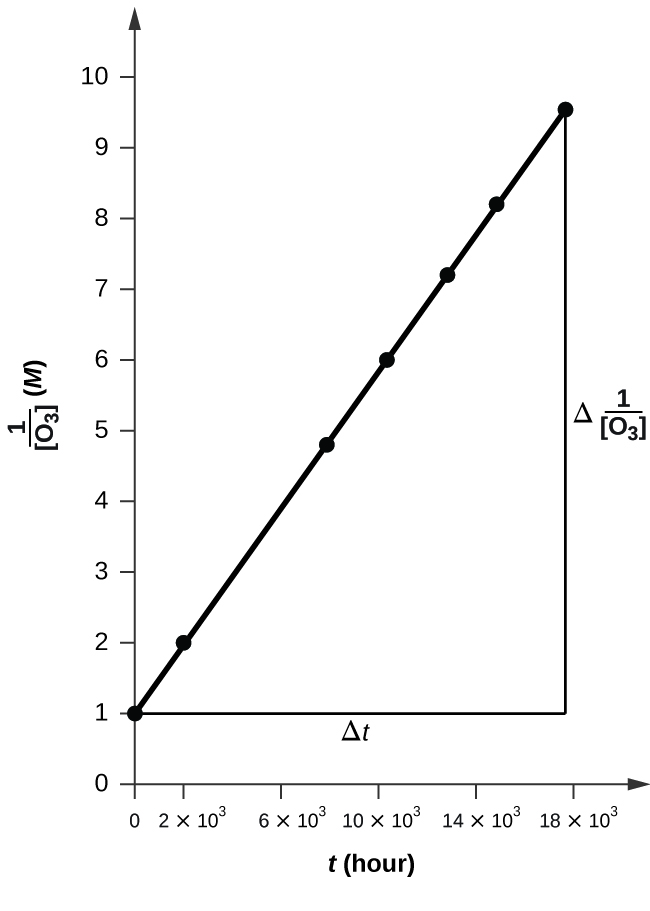



12 4 Integrated Rate Laws Chemistry



Concentration Time Relationships Integrated Rate Laws Introductory Chemistry




Chapter 12 Chemical Kinetics Ppt Download




Rates And Mechanisms Of Chemical Reactions Ppt Download
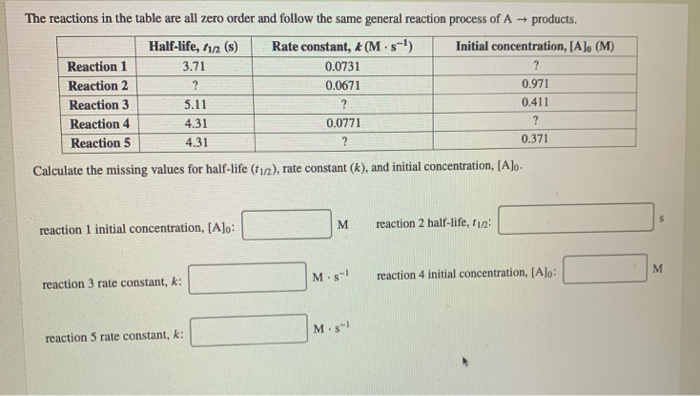



The Reactions In The Table Are All Zero Order And Chegg Com




The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png
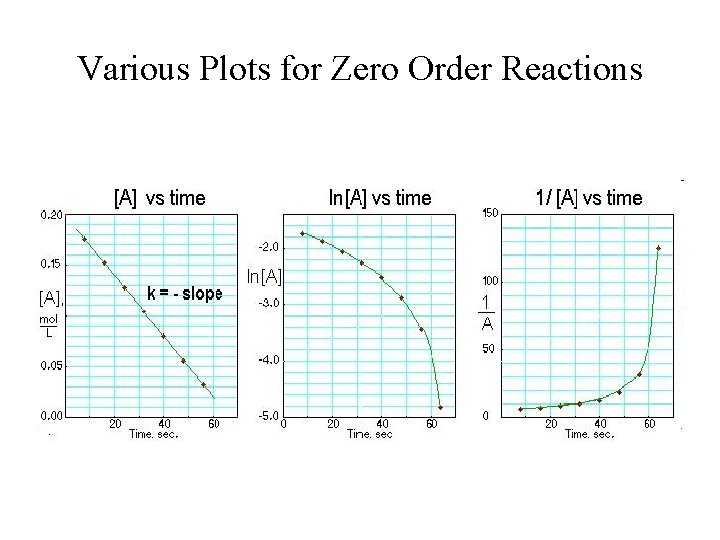



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of




14 4 Zero Order Reactions Chemistry Libretexts




Solved Question 12 1 Point Which Of The Following Are True Chegg Com
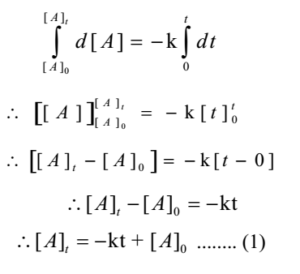



Rate Of Zero Order Reaction Integrated Law Half Life Period Rate Constant




Iit Jee Zero Order Reaction With Numerical In Hindi Offered By Unacademy




Half Life Introduction To Chemistry
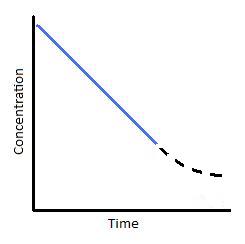



2 10 Zero Order Reactions Chemistry Libretexts




Table Of Contents 12 1 Reaction Rates Ppt Download




Half Life Ppt Download




Half Life Of A First Order Reaction And A Zero Order Reaction Are Same Then The Ratio Of The Initial Rate Of The First Order Reaction To That Of Zero Order Reaction
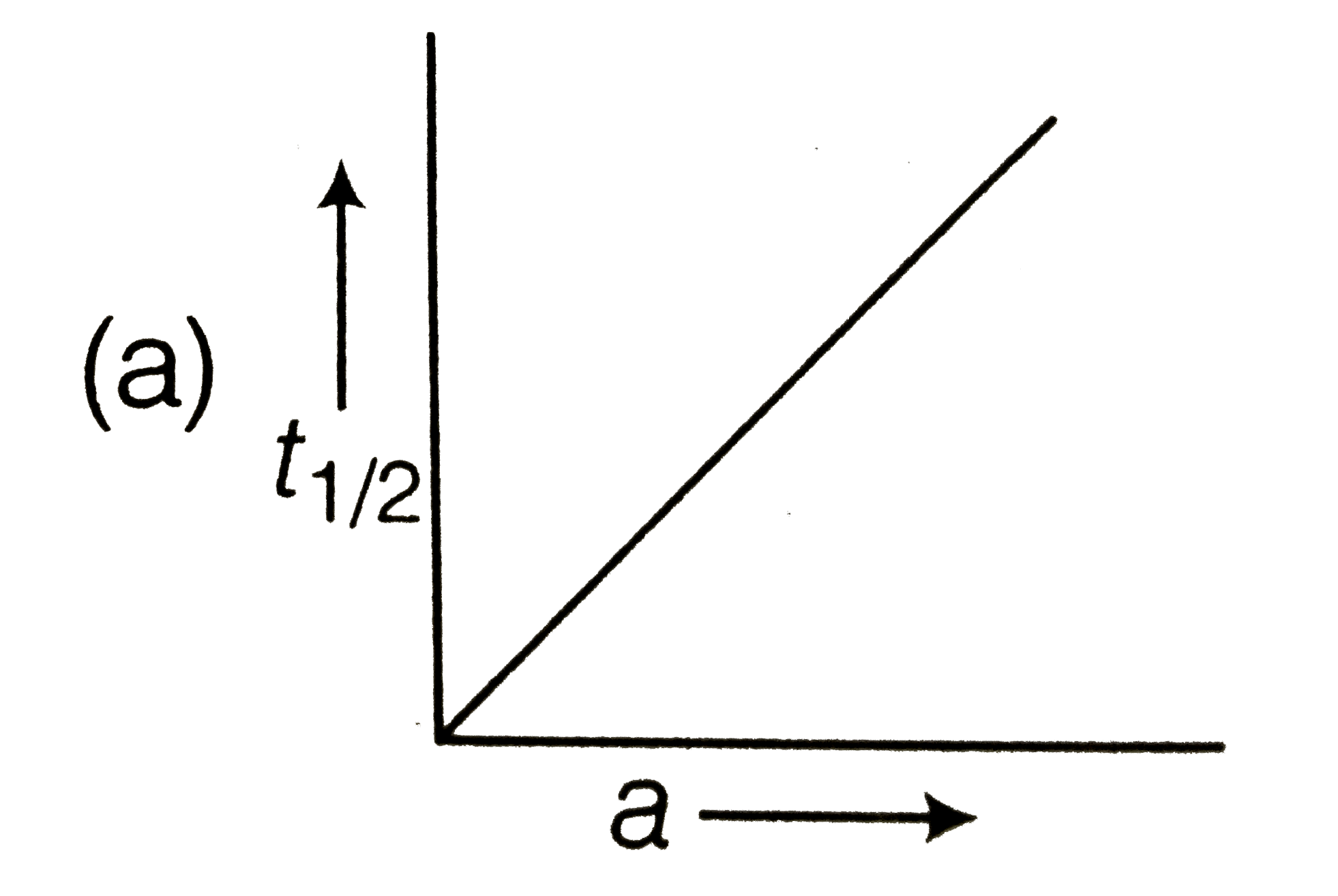



Which Of The Following Graphs Formed Plotted Between T 1 2
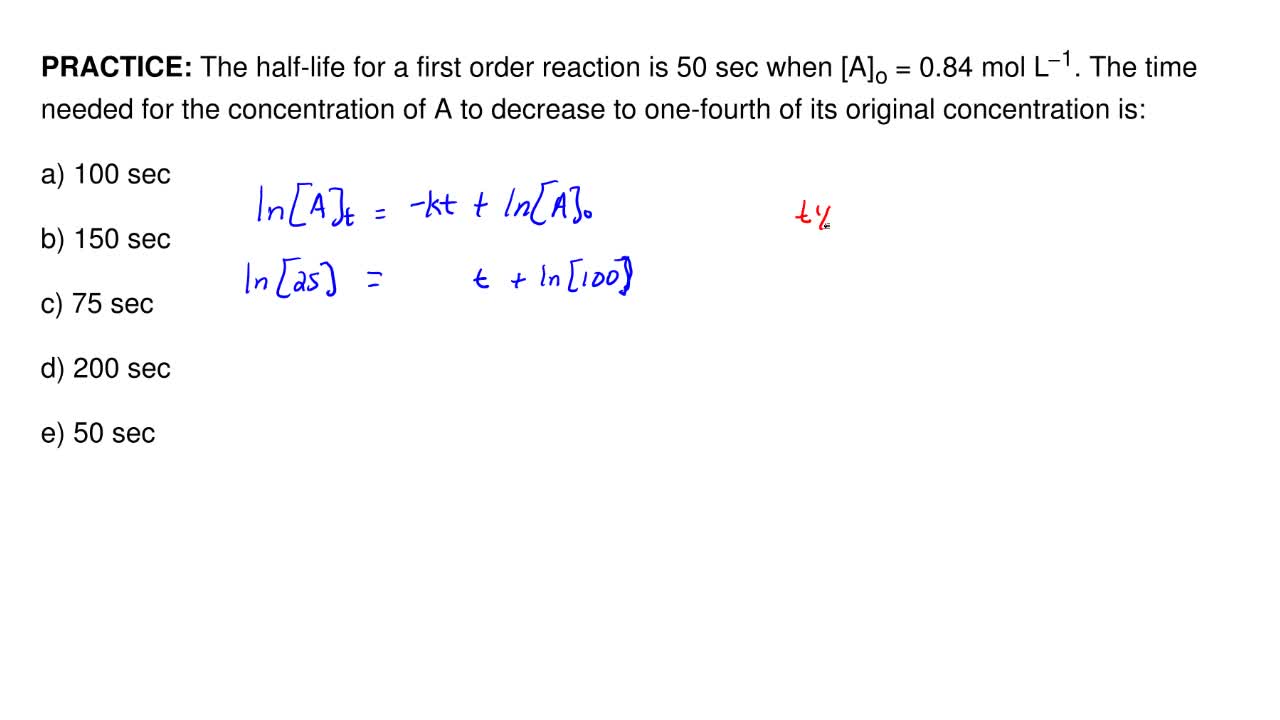



The Half Life For A First Order Reaction Is 50 Sec Wh



Integrated Rate Law
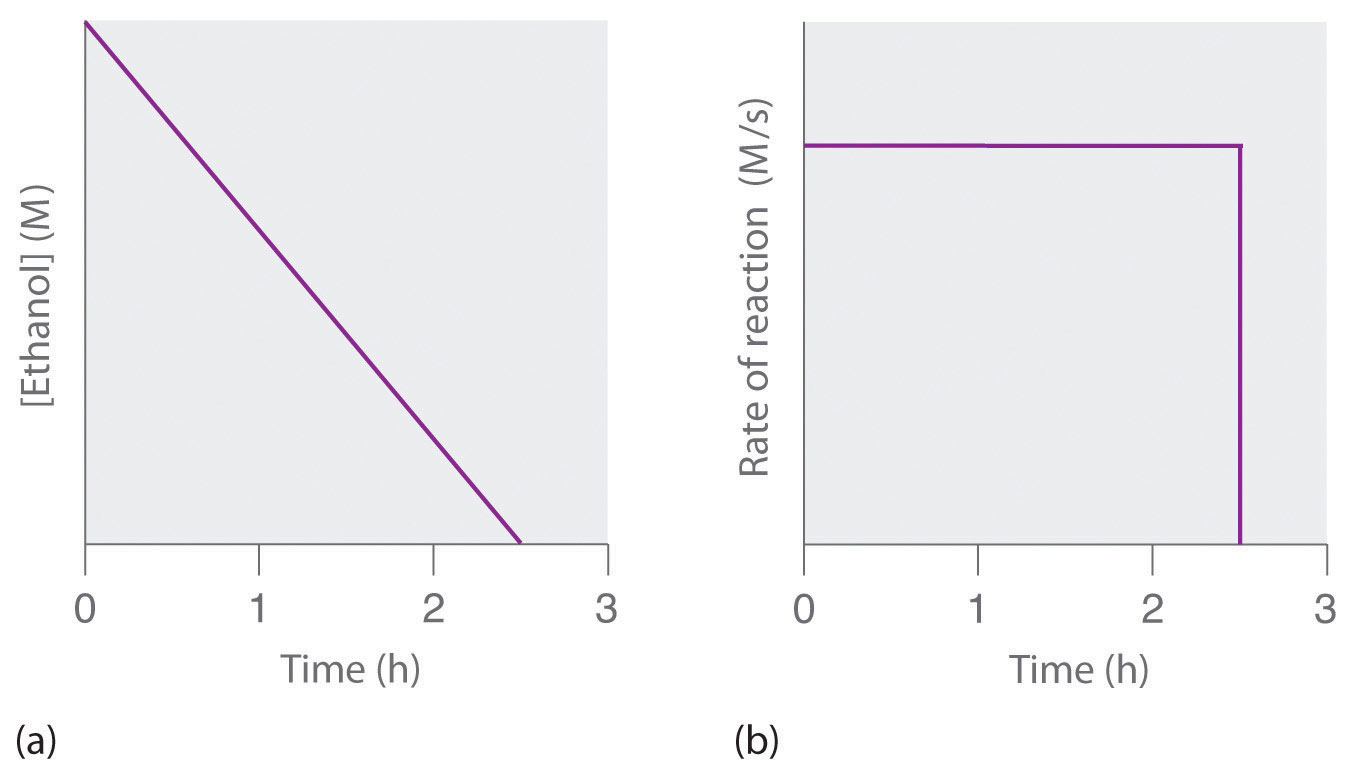



Methods Of Determining Reaction Order




For A Reaction A Arrow Products A Plot Of Log T1 2 Versus



What Is The Order If Half Life Of A Reaction Is Halved As The Initial Concentration Of The Reactant Is Doubled Quora
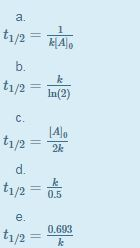



Which Of The Following Corresponds To The Correct Chegg Com



2




0 00 O El 1 0 Question 12 1 Point Which Of The Chegg Com



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of




Solved 69 Which Option Is Valid For A Zero Order Reaction A T3 4 3 12 B 12 34 C T1 2 2t314 D T3 4 T12 Aiims 1 11 Conrecents The Evnression For



Reaction Rates Chapter 13 Ppt Video Online Download




The Rate Law Concentration And Time Boundless Chemistry




Zero Order Reaction Definition Derivation Graph Examples




2 Chemistry Part Ii Pages 151 0 Flip Pdf Download Fliphtml5




Integrated Rate Equations Rate Law Zero First Order Reactions Videos



Solved Which Of The Following Represents The Equation For A Zero Order Half Life Course Hero



Half Life Ppt Download




Solved 2 Which Of The Following Relation Is Correct For Zero Order Reaction A T3 4 2t12 B T3 4 1 5 12 C T34 Tv2 D Tga Stva




Chemical Kinetics
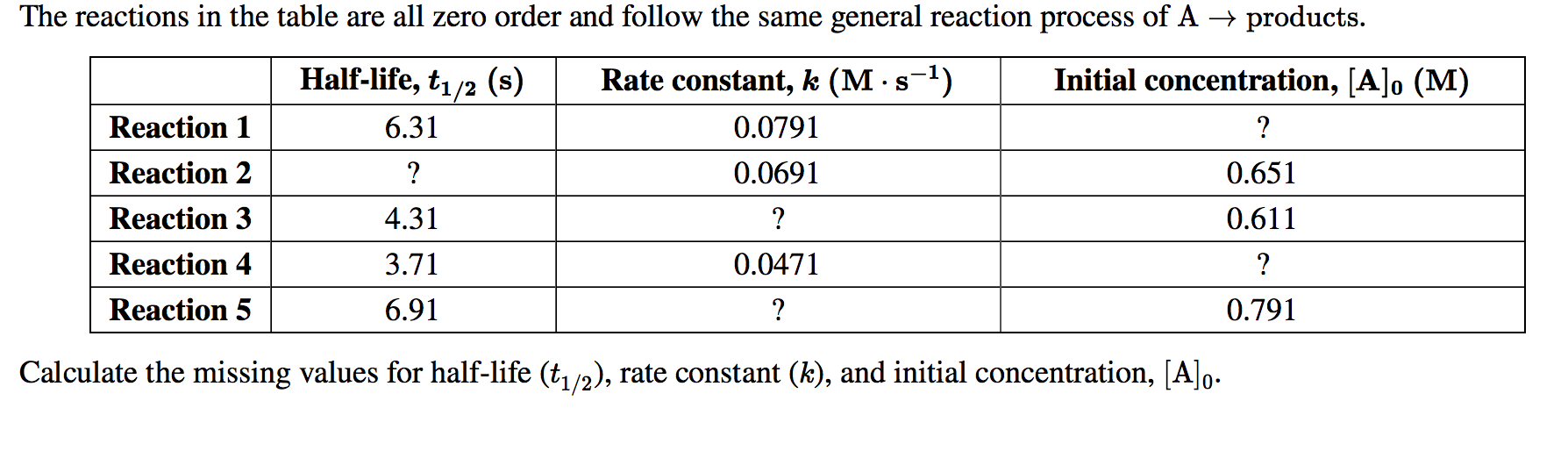



Solved The Reactions In The Table Are All Zero Order And Chegg Com




Second Order Reaction Definition And Derivation For Rate Law And Half Life



Chemical Kinetics Chemical Kinetics The Branch Of Chemistry



Order Reaction S J Shah



1




Rate Of Zero Order Reaction Integrated Law Half Life Period Rate Constant




The Plot Of T1 2 V S R 0 For A Reaction Is A Straight Line




Which Of The Following Is Not Correct For Zero Order Reaction




Determination Of Order Of Reaction Study Material For Iit Jee Askiitians



Zero Order Reaction Definition Examples Formula




Match The Graphs Given In Column I To The Parameters And Condit
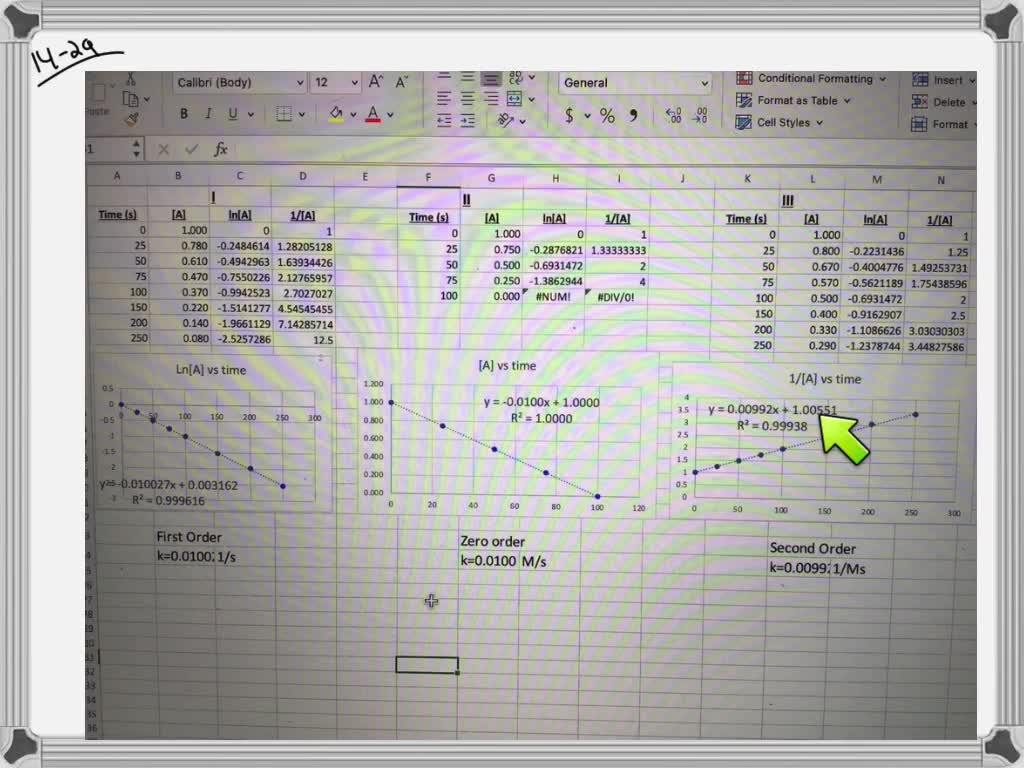



Solved The Reactions In The Table Are All Zero Order And Follow The Same General Reaction Process Of Products Products Halfa Life D 1 2 D T1 2 S Rate Constant D D œa D Aˆ 1 K Ma Saˆ 1
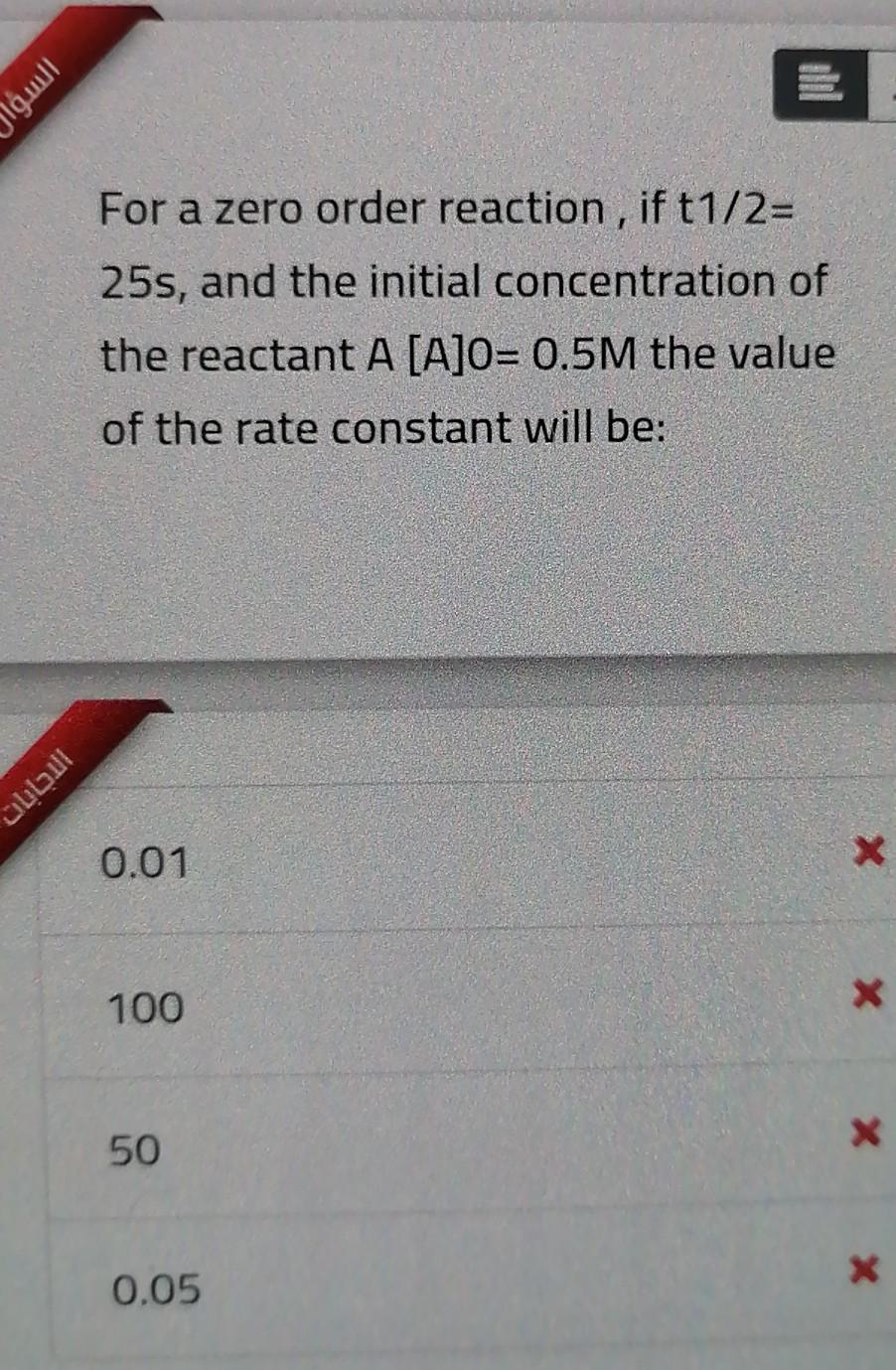



Solved السؤا For A Zero Order Reaction If T1 2 25s And Chegg Com




For Zero Order Reaction Relation Between T And T Is Brainly In
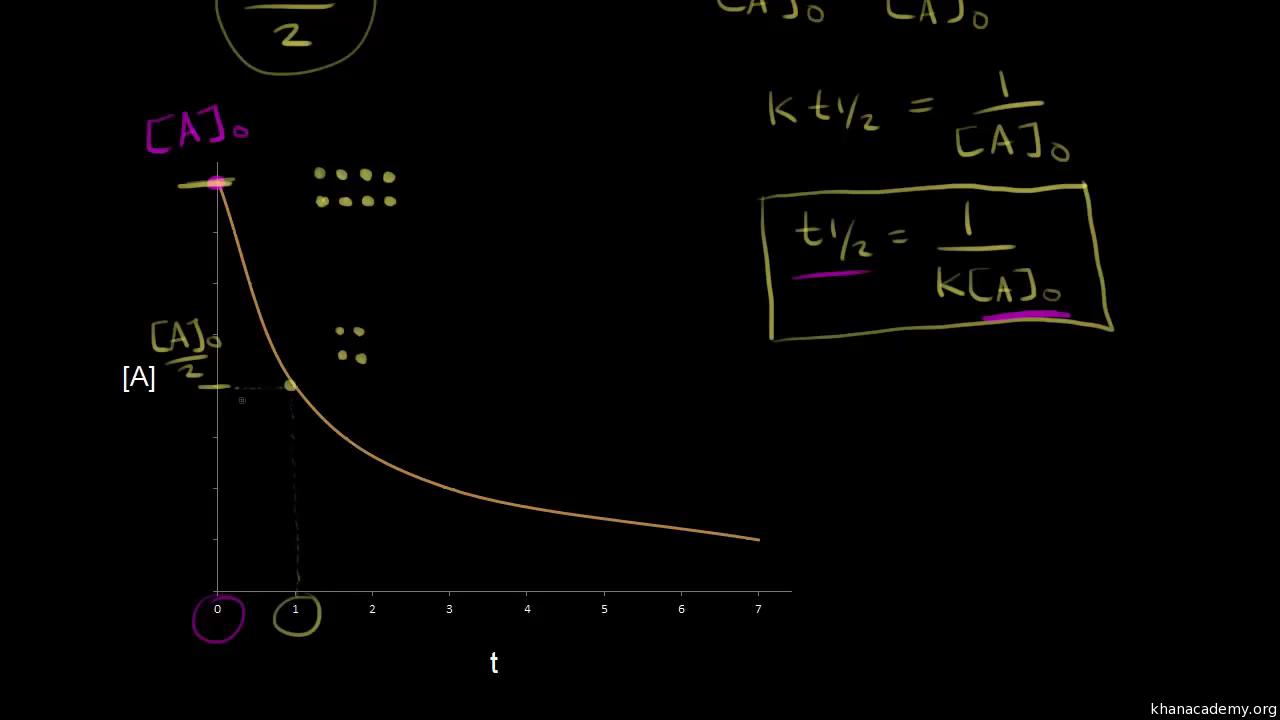



Half Life Of A Second Order Reaction Video Khan Academy




For A Second Order Reaction 2ararr Products A Plot Of Log
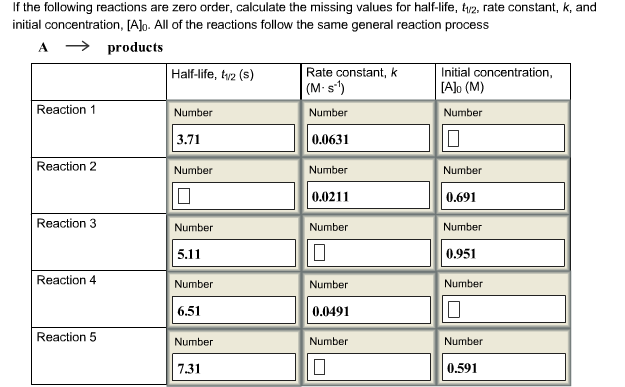



Solved If The Following Reactions Are Zero Order Calculate Chegg Com




For A Zero Order Reaction The Relationship Between T1 2 1st And T1 2 2nd Is
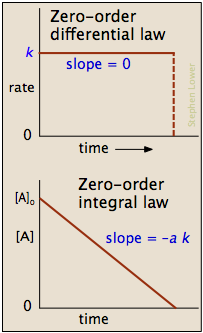



2 10 Zero Order Reactions Chemistry Libretexts




Zero Order Reactions Introduction To Chemistry
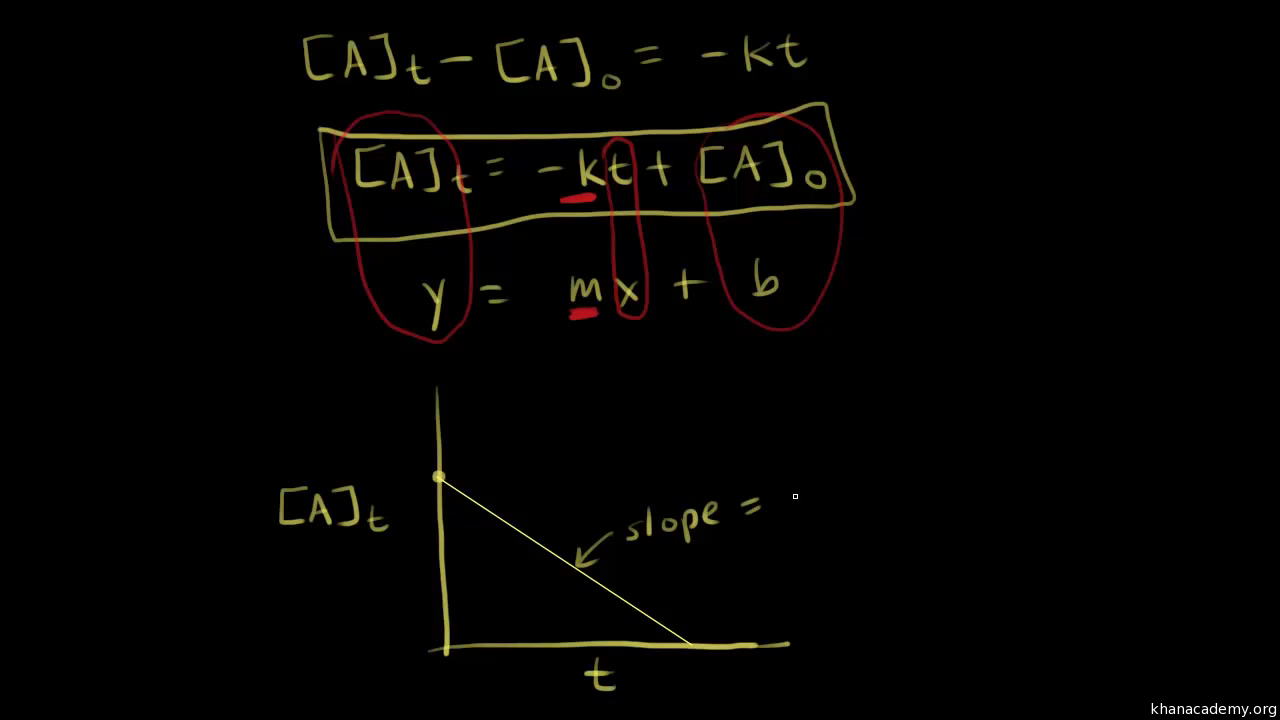



Zero Order Reaction With Calculus Video Khan Academy



The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png



Half Life Of Zero Th 0th Order Reaction Derivation Youtube
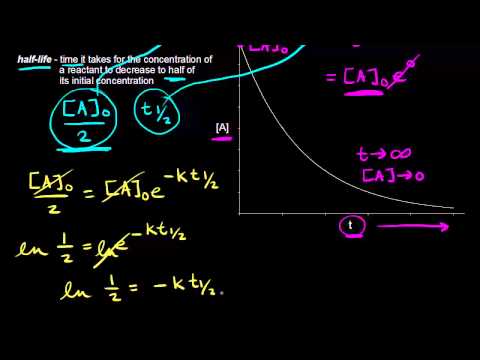



Half Life Of A First Order Reaction Video Khan Academy
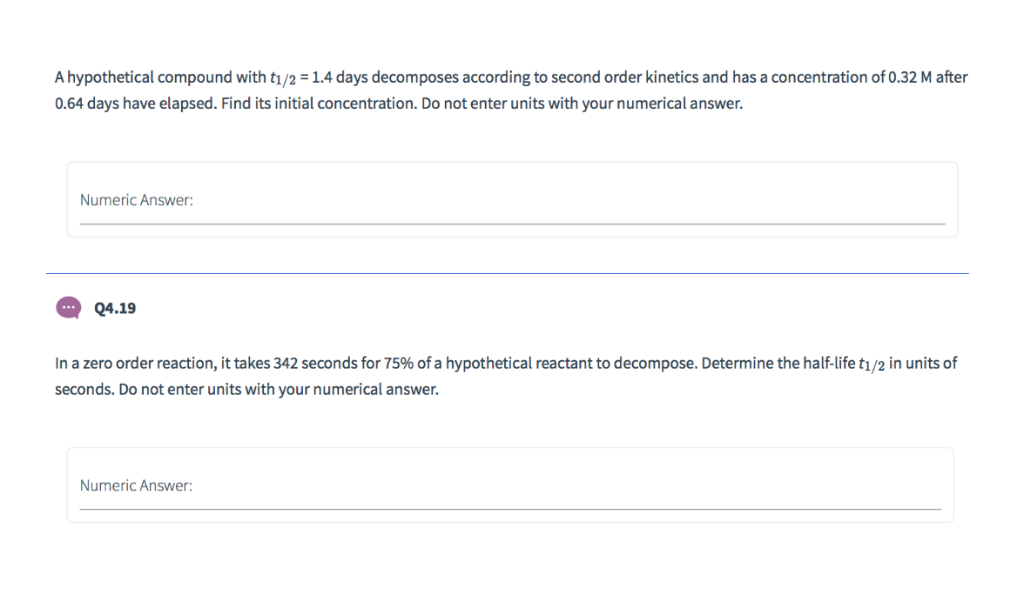



Solved A Hypothetical Compound With T1 2 1 4 Days Chegg Com




3 The T For A Zero Order Reaction At The Initial Concentrat Scholr



1



When The Concentration Of A Reaction Is Doubled Its Half Life Remain Same What Is The Order Of The Reaction Quora
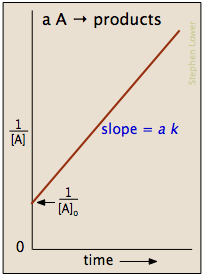



2 8 Second Order Reactions Chemistry Libretexts




Solved For A Zero Order Reaction T1 2 Is Proportional To What
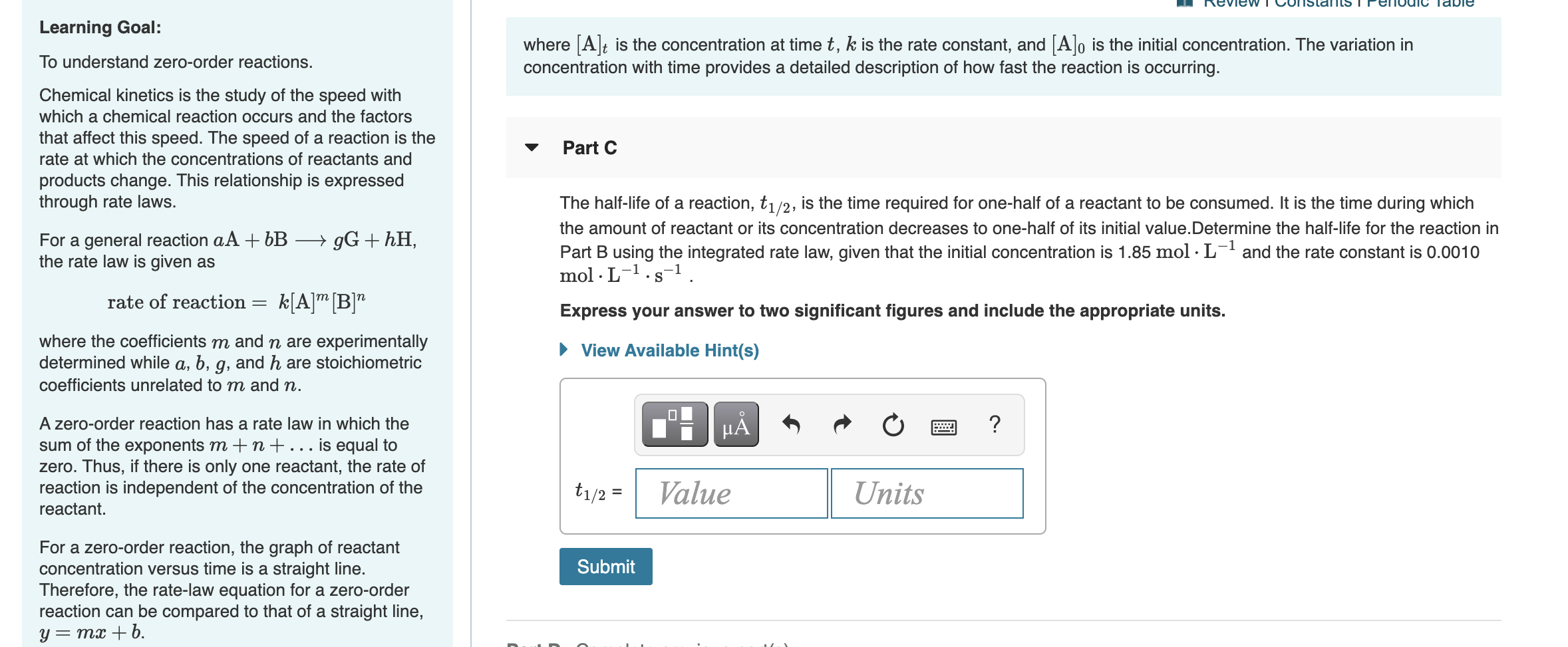



Learning Goal To Understand Zero Order Reactions Chegg Com
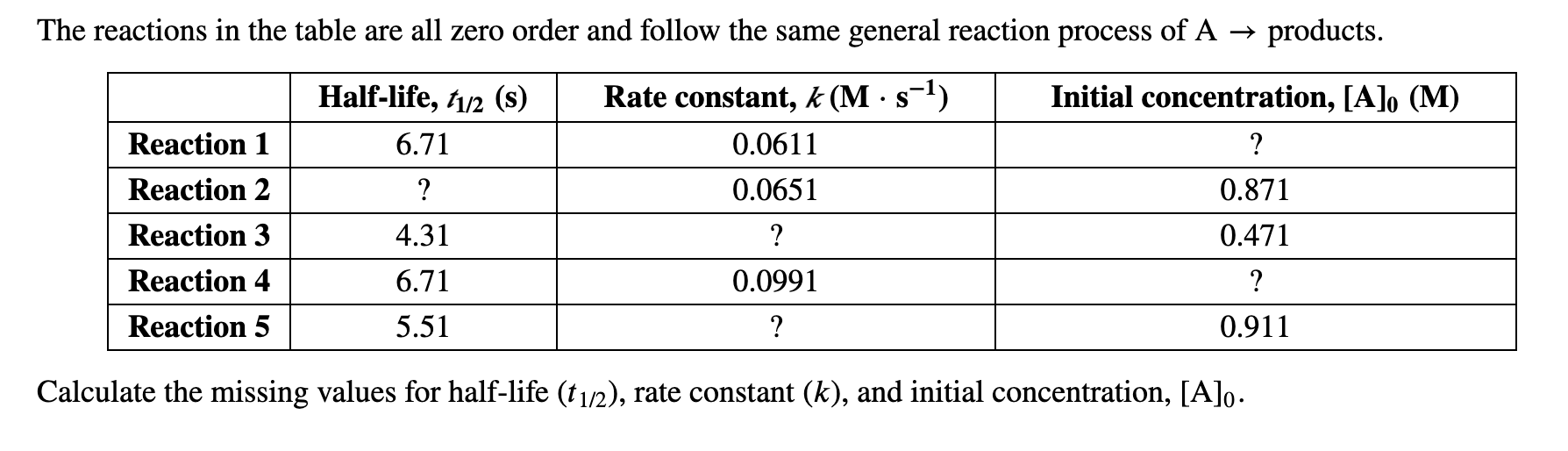



The Reactions In The Table Are All Zero Order And Chegg Com




Rate Of Zero Order Reaction Integrated Law Half Life Period Rate Constant




Solved 2 Which Of The Following Relation Is Correct For Zero Order Reaction A T3 4 2t12 B T3 4 1 5 12 C T34 Tv2 D Tga Stva



When The Concentration Of A Reaction Is Doubled Its Half Life Remain Same What Is The Order Of The Reaction Quora




Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id



2




Data Fits A Zeroth Order Reaction 13 What Data Chegg Com




Solved The Integrated Rate Laws For Zero First And Chegg Com
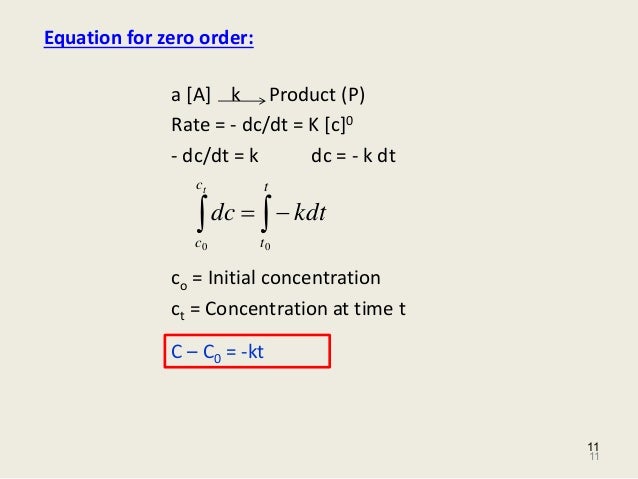



Kinetics And Drug Stability Ed
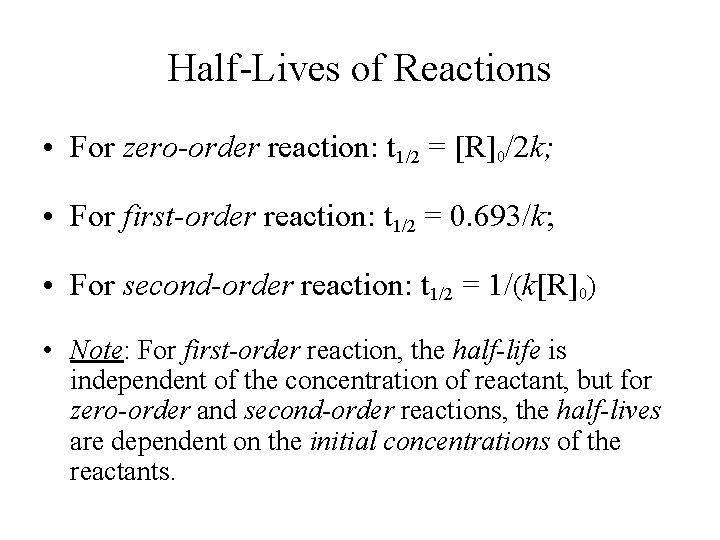



Chemical Kinetics Expression Of Rates Stoichiometric Relationships Of




Integrated Rate Equations Rate Law Zero First Order Reactions Videos
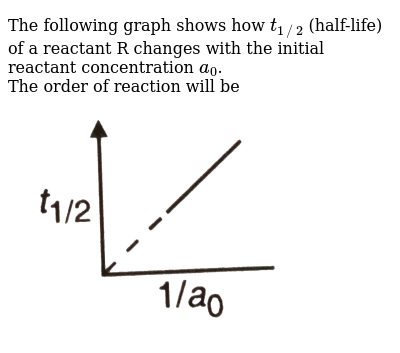



The Following Graph Shows How T 1 2 Half Life Of A Reactant R Changes With The Initial Reactant Concentration A 0 The Order Of Reaction Will Be Img Src D10lpgp6xz60nq Cloudfront Net Physics Images Din Obj Chm V01 C3 3 E01 254 Q01 Png



0 件のコメント:
コメントを投稿